EKG Challenge #4 Case Conclusion: In Syncope, You Can't Miss These
You are riding along with EMS when you get a call for "difficulty breathing". You enter the house to find fire department already on scene performing CPR on a high school age male. The paramedics give 2mg of intra-nasal Narcan without response. The patient is placed on the monitor and the following rhythm strip is obtained:
The patient is defibrillated x 2 with ROSC and his post-defibrillation strip:
After return of pulses, the patient is bagged on transport to the emergency department. An additional 2mg of narcan is given IV without effect. A 12 lead EKG is obtained:
On evaluating the EKG, you are struck by the short PR interval and up-sloping of the QRS. You review the initial rhythm strip and identify an irregular and fast (~ 300 bpm) consistent with atrial fibrillation with a wide QRS. You call Cardiology, who agrees with your diagnosis of WPW.
To review, WPW is a pre-excitation syndrome in which there is a manifest accessory pathway for conduction between the atria and the ventricles. This accessory pathway predisposes to a number of serious arrythmmias. See a nice review from Life in the Fast Lane here. Technically, WPW is a syndrome in which the congenital accessory pathway is present and patients are symptomatic secondary to episodes of tachyarrythmmia.
Once you understand the electrophysiology, the triad of ECG findings in WPW makes sense:
1) shorted PR interval because ventricular stimulation begins earlier than normal via the accessory pathway.
2) a slurred rather than sharp upstroke of the QRS (delta wave) due to ventricular stimulation through the accessory pathway.
3) a widened QRS complex which it represents fusion of two excitation wavefronts through the ventricles.
WPW is most likely to degenerate into Vtach or Vfib arrest when atrial fibrillation occurs. Atrial fibrillation occurs in up to 20% of patients with WPW, and because the accessory pathway has no refractory period for conduction like the AV node, the ventricular rate can be very high - upwards of 300 bpm [1]. The hallmarks? Look at the initial rhythm strip for this patient: irregular, bizarre-appearing, wide complexes at a rate of ~ 300 bpm.
Back to our patient...
Almost immediately after ED arrival, the patient is intubated for continued poor GCS, admitted to the ICU, where he gradually stablilizes, and is eventually discharged with normal neurologic status. Prior to discharge, he undergoes an electrophysiology study which identifies and ablates an accessory pathway, and his post-procedure EKG is notable for the absence of a delta wave:
On further review of the patient's EMR, you see that he was seen in an emergency department three years earlier after being found confused/altered. At the time, mental status was thought to be secondary to reported intake of OTC sleep meds and marijuana use. An EKG was obtained during the visit:
Review of this EKG reveals a short PR interval and evidence of a delta wave in several leads (see II, aVF), and diffuse ST changes. The patient was discharged home at that time with a diagnosis of drug abuse. Despite the number of a "normal EKGs" we see on a daily basis for patients who present with syncope, it is important to maintain vigilance for the Can't miss EKGs findings in the young person with syncope:
1. WPW: As mentioned above, you can read about WPW at Life in the Fast Lane. You can also watch Amal Mattu's episodes on WPW with atrial fibrillation and on orthodromic and antidromic SVT, or read about the emergent treatment of arrythmmias associated with WPM here.
2. Brugada syndrome: Brugada is a genetic syndrome of sudden death associated with specific electrocardiographic findings: RBBB (possibly incomplete) and ST elevation in the right precordial leads (V1 - V3). The ST elevation can have a coved or saddle-back morphology (see Figure below). Brugada is associated with cardiac arrest secondary to ventricular fibrillation and most typically presents with syncope (if the rhythm self-terminates) or sudden cardiac death, usually in the 4th or 5th decade of life [4]. Patients with suspected Brugada should undergo EP testing, as treatment with an AICD can be lifesaving. For further edification, watch Amal Mattu's ECG of the week on Brugada syndrome or read a written review published in Emergency Medicine News.
3. Long QT syndrome: A prolonged QTc interval is considered > 440 ms in males and > 460 ms in females. A basic rule of thumb is that the QT should measure < 1/2 of the RR interval. While we normally think of prolonged QT as a risk factor for cardiac arrythmmia (Torsades de Pointes) in the context of QT-prolonging medications or electrolyte abnormalities, there are a number of genetic
mutations in sodium and potassium channels that also lead to prolonged QT syndrome which can present as sudden cardiac arrest early in life. In a study of 647 patients who were carriers for one of these mutations, 87% experience cardiac arrest or death over a period of 28 years [2]. The first medical presentation for cardiac event (including syncope) is usually in the range of 10 - 35 years [2]. Because of the electrophysiologic effects of the mutations, patients are particularly susceptible to cardiac arrest during adrenergic stimulation. Beware, the computer does not always measure the QTc correctly, here is a tutorial from Life in the Fast Lane on how to measure it correctly yourself and an example from Steve Smith's ECG blog.
4. Hypertrophic Cardiomyopathy (HCM): HCM is the most common cause of sudden cardiac death in young athletes. HCM is actually a heterogeneous group of cardiac conditions due to genetic
mutations in components of the cardiac sarcomere that have variable penetrance and severity of clinical presentation. Patients can present initially with symptoms predominantly due to LVOT obstruction (exertional syncope, lightheadedness on exertion) or sudden cardiac death. The common phenotype is left ventricular hypertrophy, which is usually most pronounced in the anterior septum and can result in obstruction of the left ventricular outflow tract (LVOT). The most common EKG findings are LVH with associated ST and T wave changes. In addition, these patients can have narrow & deep Q waves in the lateral leads with a duration of < 40 ms. These reflect septal hypertrophy and are often mistaken for changes of prior ischemic event. The EKG findings of HCM can precede an abnormal echo. For an excellent summary and several good ECG examples, read the HCM review from Life in the Fast Lane.
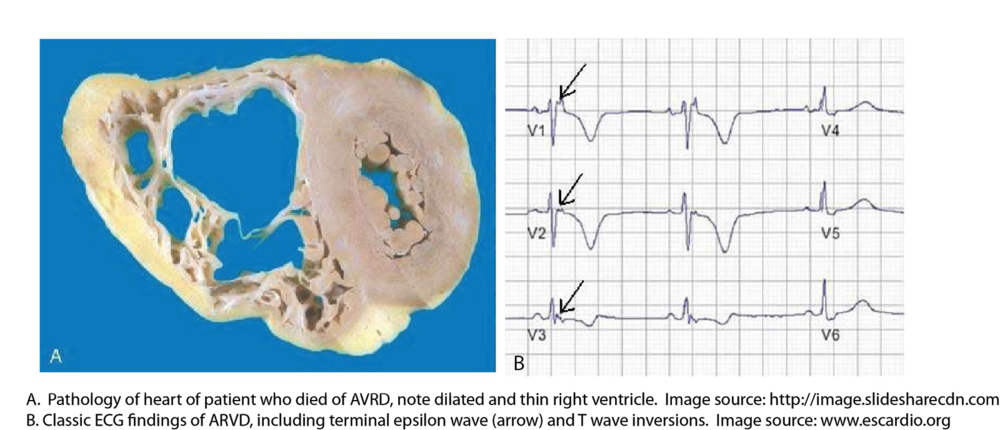
5. Arrythmogenic Right Ventricular Dysplasia (ARVD): Arrythmogenic Right Ventricular Dysplasia is a genetic disease characterized by progressive replacement of the right ventricular myocardium by fatty fibrous tissue. At the cellular level, it is a disease of desmosome dysfunction. As a reminder, desmosomes are connections between cardiac myocytes that allow them to electrically communicate with each other. ARVD has been proposed to explain 3-5% of sudden cardiac death under the age of 65 [5]. It is associated first with ventricular arrythmmias (with a LBBB pattern because they originate in the right ventricular outflow tract) leading to sudden death (80% of patients present with cardiac arrest). Those who survive eventually develop right heart failure. The EKG findings of ARVD are related to extensive scarring of and slowed conduction through the right ventricle [6]:
- Epsilon wave - a small positive deflection at the very end of the QRS complex
- T wave inversions in V1-3
- localized QRS widening and prolonged S-wave upstroke in V1-V3.
Here is another great review and case scenario for ARVD from Life in the Fast Lane. If you like to watch videos instead, see this case review from Amal Mattu.
Given the role of the ECG in diagnosing conditions that may later present as cardiac arrest, is there any role for screening ECGs in asymptomatic patients? Several other countries think so: Japan has mandatory ECG screening of all children and in Italy all children have a screening ECG before sports-participation [7]. While the ECG has a very high negative predictive value for LQTS, WPW, and HCM (in the realm of 99 - 100% depending on the condition), the positive predictive value is very low because of the overall low prevalence of disease (45 per 100,000 for WPW; 7 per 100,000 for LQTS; 136 per 100,000 for WPW), leading to a number needed to screen to detect one abnormality in the realm of 500-2000 children [8]. Given the poor positive predictive value, the overall rate of false positives is high, leading to potential unnecessary testing and anxiety. Thus, further studies will be necessary to determine whether ECG screening of asymptomatic children (and in what context) is worthwhile public health policy [8].
Take Home Points: Know the Can't miss EKG findings young syncope patients [Brugada, WPW, long QT, hypertrophic cardiomyopathy, ARVD ] and refrain from attributing altered mental status or syncope to drugs of abuse when the EKG is abnormal. While we will did not discuss them here, it goes without saying - keep vigilance for cardiac ischemia and PE in the syncope patient, even a young one.
References:
[1] Dovgalyuk, J., Holstege, C., Mattu, A., & Brady, W. J. (2007). The electrocardiogram in the patient with syncope. The American journal of emergency medicine, 25(6), 688-701.
[2] Priori, S. G., Schwartz, P. J., Napolitano, C., Bloise, R., Ronchetti, E., Grillo, M., ... & Cappelletti, D. (2003). Risk stratification in the long-QT syndrome. New England Journal of Medicine, 348(19), 1866-1874.
[3]Kelly, B. S., Mattu, A., & Brady, W. J. (2007). Hypertrophic cardiomyopathy: electrocardiographic manifestations and other important considerations for the emergency physician. The American journal of emergency medicine, 25(1), 72-79.
[4] Mattu, A., Rogers, R. L., Kim, H., Perron, A. D., & Brady, W. J. (2003). The Brugada syndrome. The American journal of emergency medicine, 21(2), 146-151.
[5]Marcus, F. I., McKenna, W. J., Sherrill, D., Basso, C., Bauce, B., Bluemke, D. A., ... & Zareba, W. (2010). Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia Proposed Modification of the Task Force Criteria. European heart journal, ehq025.
[6] Marcus, F. I., & Zareba, W. (2009). The electrocardiogram in right ventricular cardiomyopathy/dysplasia. How can the electrocardiogram assist in understanding the pathologic and functional changes of the heart in this disease?. Journal of electrocardiology, 42(2), 136-e1.
[7] Vetter, V. L. (2009). The role of ECG screening in the evaluation of risk of sudden cardiac arrest in the young. Pacing and Clinical Electrophysiology, 32(s2), S6-S14.
[8] Rodday, A. M., Triedman, J. K., Alexander, M. E., Cohen, J. T., Ip, S., Newburger, J. W., ... & Leslie, L. K. (2012). Electrocardiogram screening for disorders that cause sudden cardiac death in asymptomatic children: a meta-analysis. Pediatrics, 129(4), e999-e1010.
Submitted by Maia Dorsett (@maiadorsett), PGY-3
Faculty Reviewed by Doug Char
Thanks again to Heather Webb (@webbmd) for another great case
The patient is defibrillated x 2 with ROSC and his post-defibrillation strip:
After return of pulses, the patient is bagged on transport to the emergency department. An additional 2mg of narcan is given IV without effect. A 12 lead EKG is obtained:
On evaluating the EKG, you are struck by the short PR interval and up-sloping of the QRS. You review the initial rhythm strip and identify an irregular and fast (~ 300 bpm) consistent with atrial fibrillation with a wide QRS. You call Cardiology, who agrees with your diagnosis of WPW.
To review, WPW is a pre-excitation syndrome in which there is a manifest accessory pathway for conduction between the atria and the ventricles. This accessory pathway predisposes to a number of serious arrythmmias. See a nice review from Life in the Fast Lane here. Technically, WPW is a syndrome in which the congenital accessory pathway is present and patients are symptomatic secondary to episodes of tachyarrythmmia.
 |
| Source: Hamilton and Sanatani. http://www.rjmatthewsmd.com/Definitions/supraventricular_tachyarrhythmias.htm |
Once you understand the electrophysiology, the triad of ECG findings in WPW makes sense:
1) shorted PR interval because ventricular stimulation begins earlier than normal via the accessory pathway.
2) a slurred rather than sharp upstroke of the QRS (delta wave) due to ventricular stimulation through the accessory pathway.
3) a widened QRS complex which it represents fusion of two excitation wavefronts through the ventricles.
WPW is most likely to degenerate into Vtach or Vfib arrest when atrial fibrillation occurs. Atrial fibrillation occurs in up to 20% of patients with WPW, and because the accessory pathway has no refractory period for conduction like the AV node, the ventricular rate can be very high - upwards of 300 bpm [1]. The hallmarks? Look at the initial rhythm strip for this patient: irregular, bizarre-appearing, wide complexes at a rate of ~ 300 bpm.
Back to our patient...
Almost immediately after ED arrival, the patient is intubated for continued poor GCS, admitted to the ICU, where he gradually stablilizes, and is eventually discharged with normal neurologic status. Prior to discharge, he undergoes an electrophysiology study which identifies and ablates an accessory pathway, and his post-procedure EKG is notable for the absence of a delta wave:
 |
| post-ablation |
On further review of the patient's EMR, you see that he was seen in an emergency department three years earlier after being found confused/altered. At the time, mental status was thought to be secondary to reported intake of OTC sleep meds and marijuana use. An EKG was obtained during the visit:
1. WPW: As mentioned above, you can read about WPW at Life in the Fast Lane. You can also watch Amal Mattu's episodes on WPW with atrial fibrillation and on orthodromic and antidromic SVT, or read about the emergent treatment of arrythmmias associated with WPM here.
2. Brugada syndrome: Brugada is a genetic syndrome of sudden death associated with specific electrocardiographic findings: RBBB (possibly incomplete) and ST elevation in the right precordial leads (V1 - V3). The ST elevation can have a coved or saddle-back morphology (see Figure below). Brugada is associated with cardiac arrest secondary to ventricular fibrillation and most typically presents with syncope (if the rhythm self-terminates) or sudden cardiac death, usually in the 4th or 5th decade of life [4]. Patients with suspected Brugada should undergo EP testing, as treatment with an AICD can be lifesaving. For further edification, watch Amal Mattu's ECG of the week on Brugada syndrome or read a written review published in Emergency Medicine News.
3. Long QT syndrome: A prolonged QTc interval is considered > 440 ms in males and > 460 ms in females. A basic rule of thumb is that the QT should measure < 1/2 of the RR interval. While we normally think of prolonged QT as a risk factor for cardiac arrythmmia (Torsades de Pointes) in the context of QT-prolonging medications or electrolyte abnormalities, there are a number of genetic
mutations in sodium and potassium channels that also lead to prolonged QT syndrome which can present as sudden cardiac arrest early in life. In a study of 647 patients who were carriers for one of these mutations, 87% experience cardiac arrest or death over a period of 28 years [2]. The first medical presentation for cardiac event (including syncope) is usually in the range of 10 - 35 years [2]. Because of the electrophysiologic effects of the mutations, patients are particularly susceptible to cardiac arrest during adrenergic stimulation. Beware, the computer does not always measure the QTc correctly, here is a tutorial from Life in the Fast Lane on how to measure it correctly yourself and an example from Steve Smith's ECG blog.
4. Hypertrophic Cardiomyopathy (HCM): HCM is the most common cause of sudden cardiac death in young athletes. HCM is actually a heterogeneous group of cardiac conditions due to genetic
mutations in components of the cardiac sarcomere that have variable penetrance and severity of clinical presentation. Patients can present initially with symptoms predominantly due to LVOT obstruction (exertional syncope, lightheadedness on exertion) or sudden cardiac death. The common phenotype is left ventricular hypertrophy, which is usually most pronounced in the anterior septum and can result in obstruction of the left ventricular outflow tract (LVOT). The most common EKG findings are LVH with associated ST and T wave changes. In addition, these patients can have narrow & deep Q waves in the lateral leads with a duration of < 40 ms. These reflect septal hypertrophy and are often mistaken for changes of prior ischemic event. The EKG findings of HCM can precede an abnormal echo. For an excellent summary and several good ECG examples, read the HCM review from Life in the Fast Lane.
5. Arrythmogenic Right Ventricular Dysplasia (ARVD): Arrythmogenic Right Ventricular Dysplasia is a genetic disease characterized by progressive replacement of the right ventricular myocardium by fatty fibrous tissue. At the cellular level, it is a disease of desmosome dysfunction. As a reminder, desmosomes are connections between cardiac myocytes that allow them to electrically communicate with each other. ARVD has been proposed to explain 3-5% of sudden cardiac death under the age of 65 [5]. It is associated first with ventricular arrythmmias (with a LBBB pattern because they originate in the right ventricular outflow tract) leading to sudden death (80% of patients present with cardiac arrest). Those who survive eventually develop right heart failure. The EKG findings of ARVD are related to extensive scarring of and slowed conduction through the right ventricle [6]:
- Epsilon wave - a small positive deflection at the very end of the QRS complex
- T wave inversions in V1-3
- localized QRS widening and prolonged S-wave upstroke in V1-V3.
Here is another great review and case scenario for ARVD from Life in the Fast Lane. If you like to watch videos instead, see this case review from Amal Mattu.
Given the role of the ECG in diagnosing conditions that may later present as cardiac arrest, is there any role for screening ECGs in asymptomatic patients? Several other countries think so: Japan has mandatory ECG screening of all children and in Italy all children have a screening ECG before sports-participation [7]. While the ECG has a very high negative predictive value for LQTS, WPW, and HCM (in the realm of 99 - 100% depending on the condition), the positive predictive value is very low because of the overall low prevalence of disease (45 per 100,000 for WPW; 7 per 100,000 for LQTS; 136 per 100,000 for WPW), leading to a number needed to screen to detect one abnormality in the realm of 500-2000 children [8]. Given the poor positive predictive value, the overall rate of false positives is high, leading to potential unnecessary testing and anxiety. Thus, further studies will be necessary to determine whether ECG screening of asymptomatic children (and in what context) is worthwhile public health policy [8].
Take Home Points: Know the Can't miss EKG findings young syncope patients [Brugada, WPW, long QT, hypertrophic cardiomyopathy, ARVD ] and refrain from attributing altered mental status or syncope to drugs of abuse when the EKG is abnormal. While we will did not discuss them here, it goes without saying - keep vigilance for cardiac ischemia and PE in the syncope patient, even a young one.
References:
[1] Dovgalyuk, J., Holstege, C., Mattu, A., & Brady, W. J. (2007). The electrocardiogram in the patient with syncope. The American journal of emergency medicine, 25(6), 688-701.
[2] Priori, S. G., Schwartz, P. J., Napolitano, C., Bloise, R., Ronchetti, E., Grillo, M., ... & Cappelletti, D. (2003). Risk stratification in the long-QT syndrome. New England Journal of Medicine, 348(19), 1866-1874.
[3]Kelly, B. S., Mattu, A., & Brady, W. J. (2007). Hypertrophic cardiomyopathy: electrocardiographic manifestations and other important considerations for the emergency physician. The American journal of emergency medicine, 25(1), 72-79.
[4] Mattu, A., Rogers, R. L., Kim, H., Perron, A. D., & Brady, W. J. (2003). The Brugada syndrome. The American journal of emergency medicine, 21(2), 146-151.
[5]Marcus, F. I., McKenna, W. J., Sherrill, D., Basso, C., Bauce, B., Bluemke, D. A., ... & Zareba, W. (2010). Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia Proposed Modification of the Task Force Criteria. European heart journal, ehq025.
[6] Marcus, F. I., & Zareba, W. (2009). The electrocardiogram in right ventricular cardiomyopathy/dysplasia. How can the electrocardiogram assist in understanding the pathologic and functional changes of the heart in this disease?. Journal of electrocardiology, 42(2), 136-e1.
[7] Vetter, V. L. (2009). The role of ECG screening in the evaluation of risk of sudden cardiac arrest in the young. Pacing and Clinical Electrophysiology, 32(s2), S6-S14.
[8] Rodday, A. M., Triedman, J. K., Alexander, M. E., Cohen, J. T., Ip, S., Newburger, J. W., ... & Leslie, L. K. (2012). Electrocardiogram screening for disorders that cause sudden cardiac death in asymptomatic children: a meta-analysis. Pediatrics, 129(4), e999-e1010.
Submitted by Maia Dorsett (@maiadorsett), PGY-3
Faculty Reviewed by Doug Char
Thanks again to Heather Webb (@webbmd) for another great case