Case of the Week: Syncope and Shortness of Breath
Author: Christian Gerhart, MD
Reviewer: Sam Stringer, MD
This is a 57-year-old patient who presents with shortness of breath. His triage note is below:
“Pt arrived by EMS. Pt had a CABG 2 weeks ago, d/c 10 days ago. Pt had a syncopal episode this morning. Pt here now satting 79% on 6L NC, placed on non-rebreather and at 89%. Pt is diaphoretic.”
His vital signs on arrival are:
BP: 100/74
HR: 101
T: 98.2F
RR: 25
O2 sat: 100% on NRB mask
He reports that was doing relatively well until a few hours prior when he went to sit on his couch. He then felt very lightheaded and believes he passed out on his couch for a brief period. He was able to call EMS who noted his initial saturations to be in the 70s and he was placed on a non-rebreather. The patient was initially roomed into a general ED pod but after being examined he was transferred to the trauma and critical care pod (TCC) for closer monitoring. He reports continued dyspnea but denies any chest pain, cough, falls or recent fevers.
On exam the patient is indeed ill appearing and diaphoretic. He has a normal mental status. He is tachypneic without accessory muscle use and can speak in full sentences though struggles some. His lung exam is notable for faint crackles bilaterally, more prominent on the right. He has mild bilateral, symmetric pitting edema. He is placed on 5L NC and is saturating in the high 90s.
An EKG was performed and is shown below. There was no prior EKG available for comparison
A portable chest x ray was obtained:
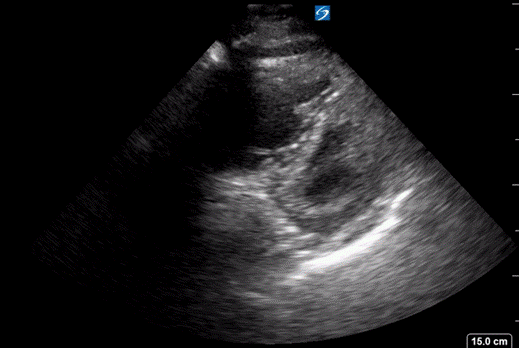
At this point a bedside ultrasound was performed. It looked something like the clip below (though this is not the patient’s US but rather images borrowed from the Everyday Ultrasound website).
What is the most likely diagnosis and what would your next steps be?
The anterolateral TWI and Q wave in III raises concern for a PE (there is also an S wave in I though the axis is normal, however there was no prior EKG to compare to. EKG findings in PE are usually not particularly sensitive but can be helpful if present. The classic S1Q3T3, generally defined as T wave inversion in lead III with both an S wave in lead I and Q wave in lead III of at least 1.5mm, had a positive likelihood ratio of 3.7 but was only present in 8.5% of patients with PE in one study.1 Interestingly, 3.5% of patients in that study had an S1Q3T3. Perhaps the most helpful EKG finding for right heart strain is T wave inversions in the anterior/inferior leads. Examining the T waves can sometimes be helpful in distinguishing between PE and ACS. One study examining patients with ACS from LAD disease and PE found that having a peak negative T wave in V1 or V2 was 96% specific for PE while the presence of TWI in BOTH lead III and V1 was also 96% specific for PE. They found that ACS generally had peak TWI in lead V3 or V4. Physiologically, this seems to make sense as V1 looks more the right side of the heart whereas V3/V4 focus more on the anterior LV myocardium. Remember, this did not include patients with ACS from other coronary artery lesions so it is unclear how this would impact the data. Interestingly, both left and right axis deviation commonly occur in PE, however right axis deviation is thought to be more associated with severe PEs. In a meta-analysis examining EKG findings in PE, tachycardia was present in 38% patients and had an odds ratio (OR) of 4.5 for hemodynamic collapse or death. 4 ST elevation in aVR, TWI in V2 or V3 also had an OR>5 for the risk of hemodynamic collapse or death.
The ultrasound demonstrated a dilated RV with decreased function and septal bowing on the PSAX view. This combined with his vital signs, history and exam was most concerning for a high-risk PE. Prior to the ultrasound the differential would be quite broad, with the other most concerning life-threatening diagnoses being cardiac tamponade, pneumonia, ACS, and new-onset heart failure. The ultrasound was key in narrowing the differential diagnosis in this case. The patient was moved immediately to the CT scanner once the ultrasound was performed. Images are shown below.
This patient had bilateral main pulmonary artery emboli with areas of the main pulmonary artery that were essentially completely occluded. He also had CT evidence of R heart strain and evidence of developing pulmonary infarctions. The ED team interpreted the CT while in the scanner with the patient and made a treatment decision shortly after.
How would you classify this pulmonary embolism? What would your next step be?
The patient’s high-sensitivity troponin returned at 40, his Pro-BNP was 2800. Lactate was 2.4. Given the information known at the time (baseline BP was unknown), this would be classified as a high risk submassive pulmonary embolism. His BPs remained in the 100s systolic while his HR was in the 100-110 range with a shock index just above 1. The fantastic chart from Josh Farkas from the IBCC below can be used as a guide for risk stratification.
The ED team activated the Pulmonary Embolism Response Team (PERT) who recommended systemic anticoagulation with unfractionated heparin and proceeding with interventional radiology embolectomy. There could be an argument for starting anticoagulation immediately once the diagnosis of a PE was thought to be “high probability” (after the ultrasound) and the patient appeared this ill. However, the time to obtain and interpret the CT at the bedside was probably only about 10-15 minutes. Additionally, as described below, there are potential dangers to aggressive anticoagulation, especially if the patient ends up needing thrombolysis. A medical ICU bed was placed. A heparin bolus and drip were started and shortly after the patient went to the IR suite. He underwent a bilateral embolectomy with improvement in his mean pulmonary artery pressures from 31 to 25 during the procedure. He went to the MICU afterwards and his vital signs improved. He was continued on heparin and was transitioned to a DOAC and discharged on hospital day 3.
Treatment of Submassive and Massive PEs:
1) Anticoagulation:
Deciding which anticoagulant to start can be challenging. Low molecular weight heparin (Lovenox) is generally easier to get to therapeutic levels and has a lower bleeding risk so is usually preferred if the patient is stable and there is a low likelihood for procedures or thrombolysis. However, in massive or submassive PEs, these patients may be going emergently for procedures or may be actively receiving thrombolysis (or may shortly receive it if they decompensate) and therefore unfractionated heparin may be preferred.17 Heparin boluses are especially prone to supratherapeutic levels which may generally be tolerated but may lead to bleeding in patients receiving thrombolytics. Here is a suggested anticoagulation strategy for submassive PEs from Josh Farkas of IBCC. The PERT Consortium notes that resumption of anticoagulation after systemic thrombolysis is not evidence based but suggests a that an unfractionated heparin infusion be resumed once the partial thromboplastin time is less than twice the normal value. (17)
2) Thrombolysis
This is a complex topic without great dosing data. Patients with a massive PE should generally get thrombolytics if no hard contraindications are present. Unfortunately, there is not a firm consensus on dosing. The American College of Chest Physicians Evidence-Based Clinical Practice Guidelines says the following:
“The quality of evidence for comparisons of systemic thrombolytic agents and regimens (eg, different doses or durations of infusion) is low based on very serious imprecision and risk of bias. In addition, there is substantial potential for publication bias. Based on this evidence, we provide only weak recommendations for all comparisons of thrombolytic agents and regimens in the short-term treatment of PE.”
With the knowledge that there are many possible dosing regimens, for a non-coding massive PE most authors recommend a 100 mg IV dose over two hours. This has been shown to be more efficacious than prolonged infusion times. (11) Depending on the patient’s stability, some authors have suggested that an initial alteplase bolus can be administered, followed by the remaining dose infused over two hours. (7,8,9) Some studies have also examined simply giving a reduced bolus dose, which did not seem to be significantly different than standard dosing strategies. (10) Typically, alteplase is still used as the thrombolytic agent of choice as opposed to Tenecteplase given that the majority of the literature has studied alteplase. Remember that once you give thrombolytics the patient will be at very high risk for bleeding. Procedures become higher risk and should be considered carefully .
A coding patient with a known PE should get thrombolytics. The dosing is again controversial in this case… a 50mg IV bolus seems well supported though some would recommend giving 100 mg as a bolus. If you choose to administer 50 mg the dose can be repeated after 15 minutes. (13,14) If alteplase is administered to a patient who goes into cardiac arrets from a pulmonary embolism, most authors recommend continuing the resuscitation for 60-90 minutes before termination. (14, 15)
Submassive PEs get even more complicated…The treatment options for a high risk submassive PE are typically anticoagulation either alone of in addition to:
Thrombolysis
- For submassive PE without a thrombolytic contraindication a “half dose” tPA strategy of 0.5 mg/kg of tPA with the first 10 mg as a bolus and a max dose of 50mg seems to be the most common regimen, which is based off of the MOPETT trial (9, 16)
- For patients with relative contraindications a dose reduction to 25mg is an option either systemically or through a catheter directed method
Embolectomy
- Can be performed if subspecialist (usually IR) available though there is not robust data on its use (17)
- Especially important to consider in patients with a thrombolytic contraindication
Remember that the patient had a coronary artery bypass surgery performed about two weeks prior. Major surgery in the past two weeks is a relative contraindication to thrombolysis
3) Pulmonary vasodilators
Works by improving hemodynamics by relieving right heart pressures and may help improve oxygenation. We have access to inhaled epoprostenol (Veletri) at our institution. Other available ones are… Alternatively, oxygen is a pulmonary vasodilator. In sick patients with a submassive or massive PE, be generous with oxygen. High flow nasal cannula is a great option for patients with respiratory distress or increasing oxygen requirement. NIPPV is an option as well, however be very cautious as positive pressure can decrease RV preload and increase RV afterload and therefore worsen or precipitate RV failure. Remember that in patients with RV failure, it is important to avoid acidemia, hypoxia, hypercarbia, and hypotension.
4) VA ECMO
There is not great data on this, but it is an option for truly unstable patients who are unable to undergo thrombolysis, or are in cardiac arrest. This can act as a bridge to thrombectomy or to keep the patient alive long enough for thombolysis to work if they have already received thrombolytics but are still unstable or arresting.
Final Thoughts:
Remember that many of these patients present after a syncopal episode. It is crucial to examine for signs of trauma before you administer thrombolytics or anticoagulation. Occasionally, there are cases where a patient will syncopize, strike their head and be appropriately diagnosed with and treated for a PE or MI but not have a trauma evaluation. Some of these patients, especially elderly patients, may have a reassuring neurologic exam initially but then their small intracranial hemorrhage expands after administration of thrombolytics or anticoagulation. Consider a head CT along with your CT PE if you have any concern that the patient struck their head.
This is one of the times when ultrasound is incredibly useful. In this case the patient was stable enough to go to the scanner with a nurse and physician since our scanner is next door. But what if he coded in the scanner? Having the information that this was likely a submassive PE with a high risk of decompensation is crucial to know earlier than later. This allows you to be prepared and have a thrombolysis or interventional plan ready if the patient decompensates.
References:
1) Marchick MR, Courtney DM, Kabrhel C, et al. 12-lead ECG findings of pulmonary hypertension occur more frequently in emergency department patients with pulmonary embolism than in patients without pulmonary embolism. Ann Emerg Med. 2010;55(4):331-335. doi:10.1016/j.annemergmed.2009.07.025.
2) Kosuge M, Ebina T, Hibi K, et al. Differences in negative T waves between acute pulmonary embolism and acute coronary syndrome. Circ J. 2014;78(2):483-489. doi:10.1253/circj.cj-13-1064.
3) Digby GC, Kukla P, Zhan ZQ, et al. The value of electrocardiographic abnormalities in the prognosis of pulmonary embolism: a consensus paper. Ann Noninvasive Electrocardiol. 2015;20(3):207-223. doi:10.1111/anec.12278.
4) Shopp JD, Stewart LK, Emmett TW, Kline JA. Findings From 12-lead Electrocardiography That Predict Circulatory Shock From Pulmonary Embolism: Systematic Review and Meta-analysis. Acad Emerg Med. 2015;22(10):1127-1137. doi:10.1111/acem.12769.
5) Chodakowski JD, Courtney DM. Pulmonary embolism critical care update: prognosis, treatment, and research gaps. Curr Opin Crit Care. 2018;24(6):540-546. doi:10.1097/MCC.0000000000000558
6) Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused [published correction appears in Am J Med. 2012 Jul;125(7):e13]. Am J Med. 2012;125(5):465-470. doi:10.1016/j.amjmed.2011.10.015\
7) Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W; Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. doi:10.1056/NEJMoa021274
8) Igneri LA, Hammer JM. Systemic Thrombolytic Therapy for Massive and Submassive Pulmonary Embolism. Journal of Pharmacy Practice. 2020;33(1):74-89. doi:10.1177/0897190018767769
9) Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the "MOPETT" Trial). Am J Cardiol. 2013;111(2):273-277. doi:10.1016/j.amjcard.2012.09.027
10) Goldhaber SZ, Agnelli G, Levine MN. Reduced dose bolus alteplase vs conventional alteplase infusion for pulmonary embolism thrombolysis. An international multicenter randomized trial. The Bolus Alteplase Pulmonary Embolism Group. Chest. 1994;106(3):718-724. doi:10.1378/chest.106.3.718
11) Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
12) Soar J, Donnino MW, Maconochie I, et al. 2018 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations Summary. Resuscitation. 2018;133:194-206. doi:10.1016/j.resuscitation.2018.10.017
13) Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: Special Circumstances of Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care [published correction appears in Circulation. 2016 Aug 30;134(9):e122]. Circulation. 2015;132(18 Suppl 2):S501-S518. doi:10.1161/CIR.0000000000000264
14) Böttiger BW, Wetsch WA. Pulmonary Embolism Cardiac Arrest: Thrombolysis During Cardiopulmonary Resuscitation and Improved Survival. Chest. 2019;156(6):1035-1036. doi:10.1016/j.chest.2019.08.1922
15) Truhlář A, Deakin CD, Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 4. Cardiac arrest in special circumstances. Resuscitation. 2015;95:148-201. doi:10.1016/j.resuscitation.2015.07.017
16) Reardon PM, Yadav K, Hendin A, Karovitch A, Hickey M. Contemporary Management of the High-Risk Pulmonary Embolism: The Clot Thickens. J Intensive Care Med. 2019;34(8):603-608. doi:10.1177/0885066618789879
17) Rivera-Lebron B, McDaniel M, Ahrar K, et al. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037. doi:10.1177/1076029619853037