REBOA: Resuscitative Endovascular Balloon Occlusion of the Aorta
By: Al Lulla, MD (EM PGY-2), Stephanie Charshafian, MD (EM PGY-4)
Faculty Reviewers: Chris Holthaus, MD (EM), Gerald Fortuna, MD (Trauma and Vascular Surgeon)
Case:
You are working your regular Friday night shift when EMS rolls in lights and sirens. From the EMS report, you gather: 28 year old male, single RUQ gunshot wound, found down by a bystander, has been hypotensive en route with systolic BPs in the 70s, coming in and out of consciousness. The patient is now moaning and diaphoretic. As your team rapidly establishes IV access, your first manual BP cuff reading is 64/40. You administer a 1L normal saline bolus and call for uncrossed blood. In the meantime, you quickly perform a FAST scan and note a positive study with fluid in Morrison’s pouch. After your second unit of blood is infusing, you check the patient’s pressure, which is only 72/44. While you are waiting for the trauma team to come down, you ask yourself, would this patient benefit from REBOA?
Clinical Question:
What is REBOA? What are the indications, contraindications and steps for placing a REBOA catheter?
Background:
Hemorrhagic shock from non-compressible torso hemorrhage (NCTH) represents a very significant cause of mortality in trauma patients. NCTH is defined as hemorrhage from trauma to torso vessels, pulmonary parenchyma, solid abdominal organs and/or disruption to the bony pelvis. It is estimated that NCTH accounts for 85% of preventable deaths on the battlefield, 80% of which include acute hemorrhage within the abdomen/torso [1]. For many decades, the gold standard to achieve hemostatic control has included operative management or interventional radiological techniques. Both require patient stability and transport time. However, when faced with cardiac arrest, sudden cardiovascular collapse, or precarious transport times do patients and providers have any other additional stabilizing options?
Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is an emerging technique that may serve as a bridge to definitive management of hemorrhagic shock. REBOA has recently evolved as an important aspect of damage control resuscitation for refractory shock and traumatic arrest that may be utilized by emergency physicians and trauma surgeons in the ED setting [2]. REBOA utilizes a balloon occlusion catheter placed via the common femoral artery (CFA) to temporarily slow bleeding from below the diaphragm. Currently two catheters exist to accomplish this purpose. The Coda® balloon catheter (ranging from 9-10 French catheters, 100-140 cm length, requiring 12-14 French sheaths) and a newer, smaller, and shorter ER-REBOATM catheter (6 French catheter, 72 cm length, 7 French sheath) FDA approved in 2015.
Emergency resuscitative thoracotomy (RT) has long been the mainstay and still remains an option in the management of patients with NCTH and subsequent hemodynamic collapse (For more details on the indications and steps of performing resuscitative thoracotomy, check out: http://www.everydayebm.org/case-based-learning/edthoracotomy). However, REBOA may offer an alternative to thoracotomy as a less invasive and less morbid approach that may provide a higher degree of safety to both the provider and the patient in certain scenarios. In animal models there is evidence to suggest that REBOA may be superior to resuscitative thoracotomy and aortic cross clamping in terms of physiological markers of resuscitation including lactic acid and pH, while having comparable improvement in cerebral and cardiac perfusion after hemorrhage from an iliac artery sheath [3].
In a 2015 human study, overall survival was shown to be greater with REBOA vs. RT (37.5% vs. 9.7%; P=0.03) when comparing REBOA (n=24) vs. RT (n=72) at two level 1 civilian trauma centers with refractory hemorrhagic shock from penetrating or blunt abdominal trauma (patients with suspected or confirmed intrathoracic injury were excluded) [4]. Additionally, there were more deaths in the ED amongst patients undergoing RT vs. REBOA (62.5% vs. 16.7%; P< 0.001). Of note, there were no differences in injury severity scores or mechanism of injury between the two groups.
Prospective data (Nov 2013-Feb 2015) from the Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry (eight ACS Level 1 centers) examined 114 patients of which 46 underwent REBOA and 68 open aortic occlusion (AO) [5]. Overall survival was 21.1% (REBOA 28.2% vs. open AO 16.1%; P=0.12) with few complications associated with REBOA (embolism 4.3%, pseudo aneurysm 2.1%, limb ischemia 0%). Hemodynamic stability (systolic blood pressure >90 mm Hg for > 5minues) if achieved occurred more often with REBOA than open AO (47.8% vs. 27.9%; P=0.01). Time to successful aortic occlusion was similar for REBOA and open AO (6.6 +/- 5.6 min vs. 7.2 +/- 15.1; P=0.84).
A recent 2016 study from a group in Japan offers a different perspective on REBOA associated outcomes [6]. From a retrospective analysis (2004-2011) of the Japan Trauma Data Bank (196 centers), blunt trauma patients who underwent REBOA and able to be analyzed (n=351) were compared to controls who did not undergo REBOA (n=1456) using a propensity matched analysis. The study found significantly lower survival to discharge in the REBOA group compared to the propensity group (26.2% vs. 51.3%; P=<0.0001). The poor outcomes reported in this study have been a highly contested point of debate. Nevertheless, several types of delays have been noted: Japanese trauma surgeons are not typically present in the hospital 24/7, REBOA treated nonsurvivors mean time to transfusion was 124 +/- 94 minutes while their mean to definitive care/OR was 172 +/- 134 minutes. The authors noted that the higher REBOA mortality may be indicative of “’last ditch efforts’ for severity not otherwise identified in the trauma registry.”
In regards to balloon occlusion times, some time is needed to allow hemostasis, clot formation, and stabilization but is weighed against longer being worse for organ perfusion. All patients will experience ischemic/reperfusion physiology following occlusion with more severe derangement seen with Zone 1 occlusion times greater than 60 minutes. Research is ongoing to evaluate partial occlusion times. Outcomes and tolerability of various balloon occlusion times may be further influenced by individual patient characteristics, co-morbid conditions, and pre-inflation hypoperfusion or low/no flow states.
Indications:
Generally speaking, REBOA can be considered in patients who are in hemorrhagic shock due to confirmed or suspected hemorrhage below the diaphragm. It’s important to note that indications vary depending on the injury patterns and the environment (i.e. combat casualty care vs. civilian trauma center) as well as the resources available at each institution (rural hospital versus tertiary care level 1 trauma center) [7]. Based on the current literature, our institution has formulated the following algorithm for REBOA deployment, which is based on the University of Maryland Shock Trauma Algorithm [4]:
Contraindications:
The only significant contraindication to placement of REBOA is suspicion or presence of intrathoracic injury, for which a resuscitative thoracotomy would likely be the preferred approach. Per the manufacturer, REBOA is also contraindicated in pregnant patients (aortic occlusion would deprive the pelvic vasculature and thus fetus of blood flow) as well as pediatric (insertion of REBOA is landmark based and has not been studied or approved for pediatrics) patient populations.
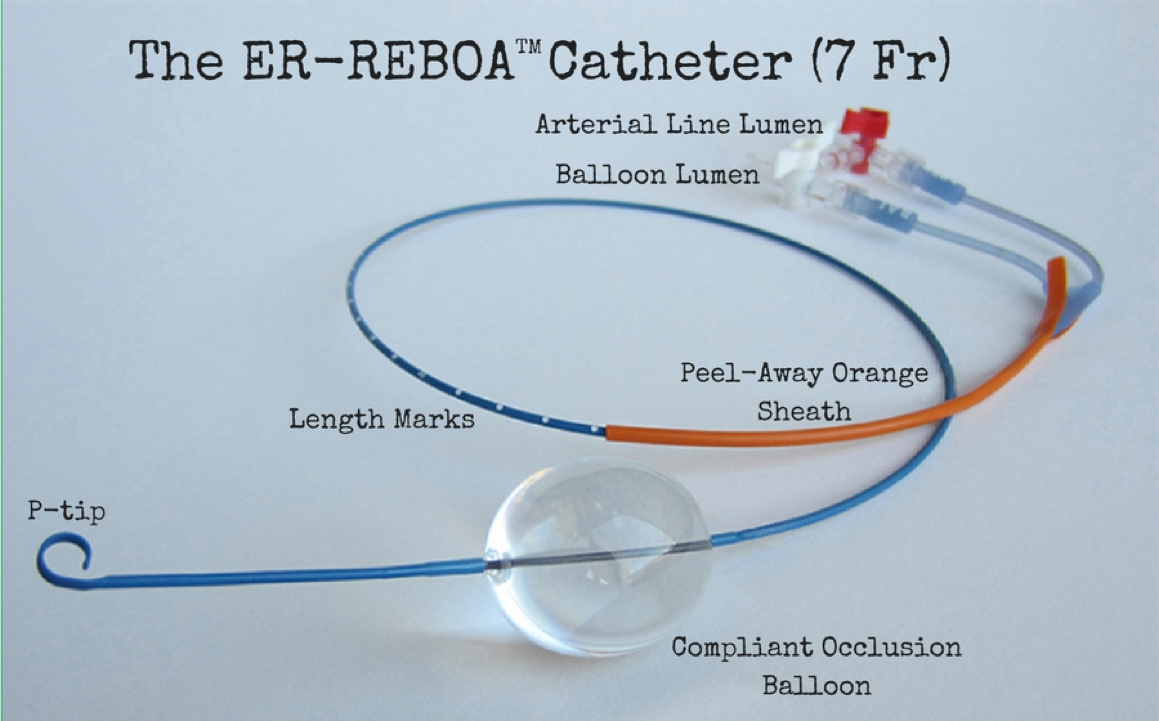
The ER-REBOA (7 Fr) Catheter (Prytime Medical):
Source: Prytime medical http://prytimemedical.com/wp-content/uploads/2017/05/er-reboa-cath-900.png
Get in the Zone [7]:
Source: Napolitano LM. Resuscitative Endovascular Balloon Occlusion of the Aorta: Indications, Outcomes, and Training. Crit Care Clin. 2017;33(1):55-70.
Prior to placing the REBOA catheter, it’s important to think about where you want your balloon to be inflated. There are three delineated zones that the balloon can occlude, zone 1, zone 2 and zone 3. For REBOA deployment, only zone 1 and zone 3 regions should be considered. Zone 1 is demarcated from the take-off of the left subclavian artery to the celiac trunk, and is ideal when abdominal hemorrhage is suspected. Zone 2 is from the celiac trunk to the lowest renal artery, and should not be considered for REBOA. Zone 3 is from the lowest renal artery to the level of the aortic bifurcation, and is optimal for controlling pelvic hemorrhage [7].
The Procedure [4,8,9]:
- Garner your personal protective equipment, including a face shield, mask, and sterile gloves.
- Establish arterial access: Via ultrasound, landmark, or cut-down access the common femoral artery (2 cm below the inguinal ligament). This can be done first pass with an arterial micropuncture kit or alternatively by using a pre-placed common femoral arterial line (ideally 18ga to allow 7 French sheath guidewire compatibility). Once in the artery, use a guidewire and Seldinger technique to advance your 7 Fr introducer sheath over the guidewire to cannulate the vessel. Note, a 7 French sheath guidewire is only compatible with an 18-gauge arterial line catheter or larger. For example, if you use a 20-gauge arterial catheter, you may not be able to thread the 7 French sheath guidewire through the catheter because the guidewire is too thick.
- Prep the catheter: Draw up normal saline in a 30 ml syringe and attach to the balloon port. Make sure to test the balloon by fully inflating and then deflating it. The balloon only holds a maximum of 24 ml of saline, and no more should EVER be inflated (please note that you will likely never need this much inflation to occlude the aorta, and insertion of more than 24 ml of saline can rupture your balloon). Attach the arterial pressure transducer to your arterial line lumen and flush your arterial line.
- Measurement: You should measure how far you will need to advance the catheter prior to inserting it. To do this, use tip of the catheter as a starting point. To occlude zone 1, place the tip of the catheter over the sternal notch and measure down to your femoral sheath. To occlude zone 3, place the tip of the catheter over the xiphoid process and again measure down to the femoral sheath. Make sure to mentally mark this on the catheter or write these numbers down in case your case evolves and you need to change from zone 1 to zone 3 (see algorithm above).
- Insert the catheter: Prior to inserting the catheter, advance the peel-away orange sheath over the p-tip to straighten it out. Insert the tip of the catheter with the orange sheath into your 7 French introducer and puncture the seal/valve. Advance the catheter approximately 10-20 cm, then slide back (or peel) the orange sheath covering the catheter to allow better visualization of the catheter depth marks as they enter the sheath. Continue to advance the catheter and stop at the sheath entrance whenyour pre-measured catheter mark/distance is reached.
- Inflate the balloon: Prior to inflation, make note of how much saline you have in your syringe and that the arterial line is reading (or ready to read). Once you open the stopcock, inflate the balloon slowly until you acquire an arterial line tracing and achieve the minimal adequate blood pressure for your patient scenario. While the balloon can theoretically accommodate up to 24 mls of saline, you should almost never need this much saline to occlude the aorta. The alleged magic balloon volume is approximately 8 mls (remember: “eight is great”). Make sure to call out and record the amount of saline instilled as well as the time when the balloon is up so an accurate assessment of ischemic time can be recorded.
- Secure your catheter, obtain an x-ray if time permits (or ultrasound perhaps TBD), otherwise keep getting on your way to definitive management (OR, IR, etc.). The clock is ticking!
Take Home Points:
- NCTH is the leading cause of death in trauma patients. REBOA offers a reasonable alternative to resuscitative thoracotomy and aortic cross clamping in patients who don’t respond to volume resuscitation, are in hemorrhagic shock, and don’t need a thoracotomy.
- Think about the possibility of REBOA early and get ahead of the curve by placing a common femoral arterial line if they are shocky (easier to do with a pulse).
- REBOA deployment in zone 1 is optimal for abdominal or any infra-diaphragmatic hemorrhage, while zone 3 deployment is better suited when pelvic or lower extremity non-compressible injury is suspected. If in doubt, consider doing zone 1 until things have stabilized and you’re definitively sure of the hemorrhagic source.
- Use the tip of the catheter and distance to your femoral access externally as a guide to measure how far you need to advance your catheter (zone 1=tip at sternal notch; zone 3= tip at xiphoid).
- Once you inflate the balloon (“8 is great”), remember more time = more ischemia. Make sure your patient is headed towards definitive management as quickly as possible.
- At this time, REBOA has generally been contraindicated in patients who have an identifiable or suspected chest/aortic injury, or in pregnant or pediatric patients.
References:
- Stein, Deborah. REBOA: Who, What and Why. https://www.youtube.com/watch?v=W8yGPeGkuJg. Accessed May 28th, 2017
- Qasim Z, Brenner M, Menaker J, Scalea T. Resuscitative endovascular balloon occlusion of the aorta. Resuscitation. 2015;96:275-9.
- White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150(3):400-9.
- Moore LJ, Brenner M, Kozar RA, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79(4):523-30.
- Dubose JJ, Scalea TM, Brenner M, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409-19.
- Inoue J, Shiraishi A, Yoshiyuki A, Haruta K, Matsui H, Otomo Y. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: A propensity score analysis. J Trauma Acute Care Surg. 2016;80(4):559-66.
- Napolitano LM. Resuscitative Endovascular Balloon Occlusion of the Aorta: Indications, Outcomes, and Training. Crit Care Clin. 2017;33(1):55-70.
- Weingart S. REBOA. Emcrit. 2015. Available at: https://emcrit.org/podcasts/reboa/. Accessed May 28, 2017.
- http://prytimemedical.com/wp-content/uploads/2017/05/ER-REBOA™-Catheter-Quick-Reference-Guide.pdf