IV lidocaine for analgesia in the ED?
Clinical scenario:
You’re in the midst of a busy evening shift, when out of the corner of your eye you think you notice a patient you just took care of a few days ago. You check the board, and sure enough you’re right. She is an unfortunate female in her 20's with a history of SLE complicated by both ESRD on dialysis and recurrent VTE. She also has chronic recurring chest pain of unclear etiology. She has had several full cardiac workups and CT imaging which have yet to reveal an etiology of her recurring pain. She tells you the pain is identical in character to her prior presentations. It is not associated with SOB, diaphoresis, lightheadedness, palpitations, or N/V. The patient is not interested in further diagnostics, and flat-out refuses admission, further CT imaging, or cardiac testing. She just wants some relief from her pain. In the past, hydromorphone at relatively high doses has been somewhat effective, but your attending is wary of continuing to provide high-dose hydromorphone at frequent intervals during your patient’s repeated ED visits. He suggests IV lidocaine may be effective for your patient.
Clinical question:
Is IV lidocaine a safe and effective parenteral analgesic alternative to narcotic pain control in the ED?
Literature review:
The analgesic properties of lidocaine in the setting of subcutaneous local anesthesia and topical anesthesia are well-appreciated. Additional uses include digital blocks, hematoma blocks, intra-articular injection, and regional nerve blocks. It is not a drug commonly used parenterally, though indications for such use do exist – both as an antidysrhythmic and for localized intravenous analgesia of an extremity exsanguinated by an arterial tourniquet (i.e., a Bier block).
In terms of systemic analgesia, the preponderance of the available literature focuses on the use of parental lidocaine for treatment of refractory neuropathic pain, particularly in the setting of advanced malignancy. There are also numerous case reports and small case series reporting its use for postoperative pain [1,6,8]. Its effectiveness in such cases is plausible based on the results of animal studies, which “suggest a link between spontaneous ectopic discharges of the injured nerve and peripheral mechanisms of neuropathic pain, and such spontaneous discharges can be suppressed by IV lidocaine in a clinically relevant dose range” [1]. Also, oral cogeners of IV lidocaine do exist (e.g., mexiletine), which could provide a possible maintenance therapy for those for whom IV lidocaine was effective. However, concerns over systemic cardiovascular and neurologic effects have thus far prevented the widespread adoption of IV lidocaine as therapy for acute pain, particularly when numerous other analgesic agents without these potential adverse effects exist (i.e., narcotics).
Double-blinded RCTs published in the 1990s first demonstrated efficacy of an IV lidocaine infusion of 5 mg/kg over 30 minutes in treating pain due to numerous neuropathic causes (including diabetic neuropathy), and postherpetic neuralgia [1,6]. One early study did not find lidocaine to be effective for pain due to a peripheral nociceptive origin (i.e., ischemic pain in a limb after blood pressure cuff inflation) [2]. The dose of lidocaine used in published studies to date ranges from 1.5 – 5 mg/kg. At these doses, lidocaine blocks function in actively depolarizing neurons without interfering with the normal function of other motor & sensory neurons [3].
Two cancer pain specialists published a review of IV lidocaine infusion for that purpose in the Journal of Supportive Oncology in 2004 [3]. In the authors’ experience, lidocaine was effective for treatment of visceral or central pain. They describe their protocol as beginning with a “lidocaine test,” consisting of a 1-3 mg/kg dose administered over 20 – 30 minutes to test for efficacy and occurrence of any adverse effects. If effective and safe, an infusion is then begun at the lowest effective dose, anywhere from 0.5 – 2 mg/kg/hr. Initially, the authors checked lidocaine blood levels, and found they were rarely over 3 mg/kg. Toxicity is rare at this dose.
Prior studies of IV lidocaine have found symptoms of toxicity to develop in a sequential and predictable manner based on blood levels [1].
Blood Lidocaine Level
|
Expected Signs/Symptoms
|
4 – 6 mg/kg
|
Lightheadedness
Perioral numbness
Dizziness
Transient HTN
|
8 mg/kg
|
Visual/auditory disturbances
Dissociative effects
Muscle twitching
Hypotension
|
12 mg/kg
|
Convulsions
|
16 mg/kg
|
Coma
|
20 mg/kg
|
Respiratory arrest
Cardiovascular collapse
|
While milder symptoms of toxicity are easily and quickly reversed by stopping the infusion and providing other appropriate supportive care, cardiovascular collapse due to lidocaine toxicity can be rapidly fatal. It is not reversible simply with infusion cessation and often requires lipid emulsion therapy.
In terms of dosage and safety, our faculty toxicologist reviewer had this to say:
"As far as the safety goes, Goldfrank's goes up to 6 mg/kg... However, I was always taught 4-4.5 mg/kg was the toxic amount. I'd be hesitant to go above that. Anecdotally, it probably should be said that we think nothing of giving approx 1.5 mg/kg to ED patients pre-intubation or with a ventricular tachyarrhythmia and do it quite safely. In fact, in ACLS we can give lidocaine twice, so up to 3 mg/kg. As such, I'm not sure why this would be considered unsafe in a pain patient."
A systematic review and meta-analysis of parenteral use of local anesthetics for treatment of neuropathic pain found IV lidocaine to be superior to placebo [6]. The weighted mean difference (WMD) of post-treatment pain ratings along a 100mm visual analog scale was -10.02mm (95% CI -16.51 to -3.54mm, p < 0.002) for those patients receiving IV lidocaine. The combined N of the included studies was 129 patients. The authors also found five trials (total N 206) which compared IV lidocaine (or its oral cogeners) to other drugs – carbamazepine, gabapentin, amantadine, and morphine. There was no significant difference in effect on pain scores, with WMD -0.60mm (95% CI -6.96 to +5.75mm).
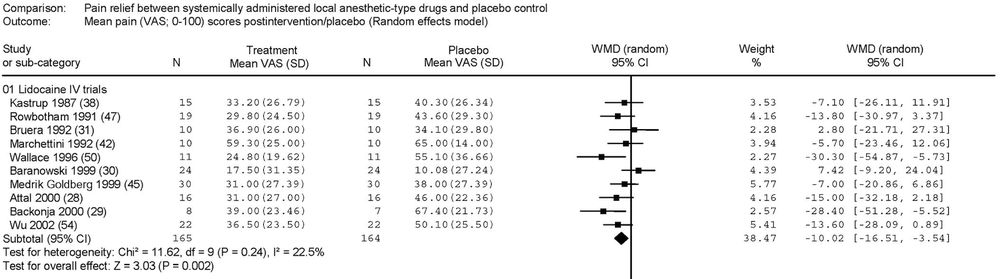 |
| From Tremon-Lukats IW, et al. Anesth Analg. 205;101:1738-49. |
 |
| From Tremon-Lukats IW, et al. Anesth Analg. 205;101:1738-49. |
There were more adverse events associated with lidocaine than with placebo, 32.2% vs 11.5%, for an OR of 4.16 (95% CI 2.68 – 6.46). The most common adverse reaction was dizziness, affecting almost 30% of treated patients. Other common reactions included nausea, vomiting, abdominal pain, diarrhea, and perioral numbness. Less frequent side effects were metallic taste, tremor, dry mouth, insomnia, allergic reactions, and tachycardia. There was no significant difference in adverse events reported between IV lidocaine and the other active drugs listed above. Of note, 410 patients were treated with IV lidocaine in the included studies (most commonly at a 5 mg/kg dose), and no serious cardiac dysrhythmia or hemodynamic instability was reported.
Another use for which there is published efficacy of IV lidocaine is for perioperative pain. A systematic review of RCTs comparing perioperative IV lidocaine vs placebo for postoperative pain found significant reductions in postoperative pain scores and opioid consumption [7]. This effect was most pronounced in open laparotomy or laparoscopic abdominal surgeries, for which the authors were able to perform a meta-analysis of 6 RCTs including a total of 250 patients. Among these patients, pain scores at 24 hrs post-op (on a 10-point scale) were significantly lower for those patients that received perioperative IV lidocaine, with a WMD of -5.93 (95% CI -9.63 to -2.23). This effect held true at rest, with movement, and with coughing, for up to 48 hours post-op. Opioid consumption was also reduced up to 85%.
There was significant heterogeneity in the dosages of lidocaine given, though most studies utilized a perioperative infusion rate of 2 – 3 mg/kg/hr. Of note, all of these trials included patients receiving both IV lidocaine and opioids, demonstrating the safety of the co-administration of these agents as no significant adverse events were reported in 395 treated patients. Four patients receiving perioperative IV lidocaine infusions did experience transient bradycardias with preserved stable hemodynamics.
A small case-series published in 2008 reported efficacy of a perioperative IV infusion of 4 mg/min of lidocaine in controlling pain for patients undergoing orthopedic surgery that were unable to be provided the usual regional analgesia for their procedures [8]. The authors report that postoperative pain scores were lower, opioid requirements from a PCA device were reduced, and hospital LOS were shorter for these patients compared with their usual experience in patients undergoing similar procedures.
A Cochrane Review (updated October 2014) investigated the safety & efficacy of IV lidocaine as a means of procedural pain relief in burn patients [4]. The Cochrane group identified only a single eligible study of 45 patients comparing IV lidocaine vs placebo. It was a randomized, double-blind, placebo-controlled, cross-over study. Increase in subjective pain ratings during procedures (e.g., wound care) was lower for the treatment arm. However, no significant clinical or statistical differences were found in terms of opioid requests or consumption, anxiety, or level of satisfaction. This small study was likely underpowered to detect such differences.
Unfortunately, none of these studies are directly applicable to the ED setting. The only study of IV lidocaine analgesia used in the ED setting comes from a study group in Iran [5]. This group studied IV lidocaine vs IV morphine in a prospective, randomized, double-blind clinical trial with 240 patients presenting with renal colic. Initial inclusion into the study was undertaken on the basis of clinical diagnosis (namely appropriate clinical picture and microscopic hematuria on urinalysis), which was later confirmed in all included patients via KUB or ultrasound. Patients were aged 18 – 65 and did not have history of renal, hepatic, or coronary disease.
All patients were initially given 0.15 mg/kg IV metoclopramide. The treatment group was given slow push of 1.5 mg/kg lidocaine dose, with control group receiving slow push of 0.1 mg/kg morphine. The study protocol was considered completed with documented pain scores of < 3 for 30 minutes, or max dose administration of 200mg lidocaine or 10mg morphine. Visual analogue scale pain scores were collected at 5, 10, 15 and 30 minutes after injection.
There were no significant differences in gender, age, prior history of nephrolithiasis, incidence of hydronephrosis, or initial pain scores between the two groups. VAS scores were significantly lower in the lidocaine group across all timepoints. The authors report that 90% of patients in the lidocaine arm and 70% of patients in the morphine arm had a “successful response” (p = 0.0001), though unfortunately they do not describe what criteria they used to determine a successful response. The study authors report that all side effects were “mild and temporary.” In the lidocaine group, 22.5% of patients experienced at least one side effect, consisting of perioral numbness, transient dizziness, and dysarthria. In the morphine group, 23.3% of patients experienced at least one side effect, consisting of vertigo, nausea/vomiting, and hypotension.
Limitations of this study include lack of true placebo arm, small sample size without description of power calculation or other true comparative statistics, lack of clear “gold standard” for diagnosis, and unclear description of primary outcome.
This same group of authors attempted to perform a systematic review of parenteral lidocaine use in the ED [9]. They found evidence for efficacy of IV lidocaine in treatment of renal colic, intractable cancer pain, chronic/refractory headache, and post-herpetic neuralgia. This was largely a descriptive study which simply listed relevant citations and general conclusions from the available literature with no quantitative analysis. Also, despite the study’s stated objectives, the authors inexplicably described multiple studies that included non-ED patients.
Take-home:
- Study of IV lidocaine as acute analgesia in the ED is extremely limited.
- Risk of cardiovascular and neurologic adverse effects cause EPs to be more hesitant in using it compared with opiates, although studies of IV lidocaine in other settings have demonstrated its safety.
- IV lidocaine appears to be most effective in treating neuropathic and visceral pain, with indications of possible efficacy for musculoskeletal pain.
- It may be reasonable to consider a low-end dose of IV lidocaine (≤ 2 mg/kg) in patients with severe neuropathic or visceral pain not adequately controlled with opiates.
- Of note, use of IV lidocaine may be restricted at your facility and may not be allowed for parenteral analgesia.
References:
[1] Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000;87(1):7-17.
[2] Bath FW, Jensen TS, Kastrup J, et al. The effect of intravenous lidocaine on nociceptive processing in diabetic neuropathy. Pain. 1990;40:29-34.
[3] Ferrini R, Paice JA. How to initiate and monitor infusional lidocaine for severe and/or neuropathic pain. J Support Oncol. 2004;2:90-4.
[4] Wasiak J, Mahar PD, McGuinness SK, et al. Intravenous lidocaine for the treatment of background or procedural burn pain. Cochrane Database Syst Rev. 2014 Oct 17;10:CD005622. [Epub ahead of print].
[5] Soleimanpour H, Hassanzadeh K, Vaezi H, et al. Effectiveness of intravenous lidocaine versus intravenous morphine for patients with renal colic in the emergency department. BMC Urology. 2012:12(13). [Epub ahead of print].
[6] Tremon-Lukats IW, Challapalli V, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetics to relieve neuropathic pain: A systematic review and meta-analysis. Anesth Analg. 2005;101:1738 –49.
[7] McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery, a systematic review of randomized controlled trials. Drugs. 2010;70(9):1149-1163.
[8] Clarke C, McConachie I, Banner R. Lidocaine infusion as a rescue analgesic in the
perioperative setting. Pain Res Manage. 2008;13(5):421-423.
[9] Golzari SE, Soleimanpour H, Mahmoodpoor A, Safari S, Ala A. Lidocaine and pain management in the emergency department: a review article. Anesth Pain Med. 2014 February;4(1):e15444 [ePub ahead of print].
Submitted by Aurora Lybeck, PGY-3.
Edited by C Sam Smith (@CSamSmithMD), PGY-3.
Faculty reviewed by Evan S Schwarz, MD FACEP (@TheSchwarziee)